To help us enhance our site, we use cookies for analytics; your data is never sold.







Metagenics UltraFlora Intensive Care Probiotic
Please log into your patient account below, otherwise you can create an account.
Microbiome Recovery Formula
New look, same formula
Microbiome Recovery Formula
Metagenics UltraFlora Intensive Care
Available in: 30 VegeCaps (15-30 days) & 60 VegeCaps (30-60 days)
Metagenics UltraFlora Intensive Care Summary:
- Rebuild native commensal flora populations.
- Improve microbiome composition, function of healthy gut flora and gut barrier integrity.
- Well-researched strains and strengths with clinically demonstrated patient health outcomes.
- Part of the Metagenics Clinical Detoxification Health Reset Program.
- Part of the Metagenics Gut Pathogen Elimination Program.
Metagenics UltraFlora Intensive Care is free from animal products, dairy protein, lactose, eggs, gluten, wheat, nuts and soy protein.
Metagenics UltraFlora Intensive Care is free from artificial colours, flavours and preservatives.
Metagenics UltraFlora Intensive Care AUST L 286746
Product Video
Metagenics How To Choose The Right Probiotic
Metagenics Clinical Detoxification Programs
Metagenics Gut Pathogen Elimination Program
Directions
Adults:
To rebuild healthy gut flora balance:
Take 1 capsule twice daily.
To assist gut health:
Take 1 capsule daily.
Take Metagenics UltraFlora Intensive Care at least two hours away from antibiotics or herbal antimicrobials.
Benefits
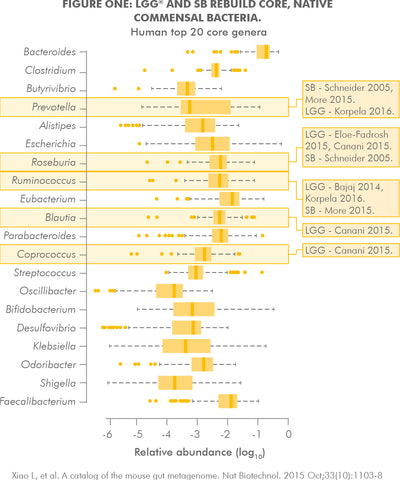
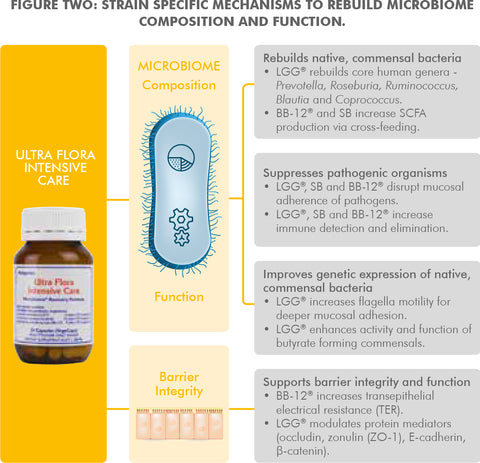
- Rebuilds native commensal flora of the gut microbiome. Recent probiotic research11 has shown that the key mechanism by which probiotics rebuild a disrupted microbiome is the modulation of native commensal bacterial populations. This is in contrast to the old understanding that probiotics repopulate by directly replacing and recolonising with specific probiotic strains and species.12,13 The dominant bacterial families of the microbiome contain over 1000 species and many thousands of genetically unique strains to totalling approximately 38 trillion organisms.14
- UltraFlora Intensive Care contains three of the world's most widely researched probiotics (LGG®, SB and BB-12®) with documented outcomes and mechanisms for microbiome replenishment.15,16 These genetically unique probiotics support the growth and function of the dominant bacterial groups of the microbiome17,18 and suppress pathogen growth.19 In addition BB-12®20 and LGG® 21 strains specifically support the gut epithelial mucosa where the bacterial populations reside and interact systemically.
- UltraFlora Intensive Care provides LGG®, SB and BB-12® strains at a clinically validated dose for microbiome restoration and to assist gastrointestinal function.22,23,24 In addition, there is a substantial supportive body of evidence showing clinical improvement in patients with symptoms associated with microbiome depletion.25,26,27
- Contains live, original and clinically researched probiotic forms. LGG®, SB and BB-12® are three of the world's most extensively researched probiotics with a range of clinically demonstrated health benefits. Factors that can influence probiotic activity include its genetic profile, manufacture (from culture to production) and storage. Live SB synthesises and secretes polyamines to exert its beneficial effects on the gastrointestinal mucosa, and inhibits pathogens.28 UltraFlora Intensive Care guarantees live SB CFU (colony forming units) at the label dose until expiry for clinical efficacy. LGG® is a genetically unique probiotic grown to a specific culturing and manufacturing process in order to provide reproducible and consistent clinical benefits in line with its wide body of research. Probiotics are genetically unique at a strain level and health benefits are not transferable between strains (even within species).
Ingredients
| Each capsule contains: | |
| 22.5 billion live probiotic organisms: | |
| Lactobacillus rhamnosus (LGG®) | 10 billion CFU (organisms) |
| Saccharomyces cerevisiae (boulardii) | 7.5 billion CFU (organisms) |
| Bifidobacterium animalis ssp lactis (BB-12®) | 5 billion CFU (organisms) |
Metagenics UltraFlora Intensive Care is free from animal products, dairy protein, lactose, eggs, gluten, wheat, nuts and soy protein. Metagenics UltraFlora Intensive Care is free from artificial colours, flavours and preservatives.
Warnings
Not all cautions and contraindications are listed. For full details, references or more information contact HealthMasters in Australia by email: reception@healthmasters.com.au.
Always read the label. Use only as directed. If symptoms persist consult your healthcare professional.
Storage
Store at 2°C to 8°C. (Refrigerate. Do not freeze.)
Online ordering is available 24/7.
Your order may ship in multiple packages to ensure they reach you as soon as possible.
If any of your products are on backorder and you are within Australia, these items will ship separately as soon as they become available at no additional cost. For international orders, your order will be sent in one package when all stock is available.
The stock availability dates are provided by our suppliers, and we endeavour to keep this information as up to date as possible on the product page.
| Within Australia | |
| Orders over $150 | $10 Express * |
| Orders under $150 | $15 Express * |
All values are Australian dollars, incl. GST.
* Deliveries to PO Boxes will be shipped standard, 2-5 days transit. Cold goods cannot be shipped to PO Boxes.
For more information, including estimated transit times and international pricing, see our Shipping page.
