Metagenics Metagen Activated B's & Folate (Metagen Methyl-Active) Technical Information
Nutrients That May Assist
- Serine
- Vitamin B6
- Pyridoxal 5-phosphate
- Vitamin B2
- Riboflavin 5-phosphate
- Vitamin B12
- Co-methylcobalamin
- Folate
- Levomefolate calcium (5-methyltetrahydrofolate)
Actions
- Supports DNA replication and genomic stability
- Supports optimal epigenetics
- Supports neurotransmitter production
Clinical Applications
- Management of hyperhomocysteinaemia
- Stress and mood support
- Cardiovascular support
Dosing Considerations*
- Pregnancy
- Breastfeeding

*Dosing regimens should be determined by appropriate assessment and monitoring.
Methylation is a chemical reaction involving the attachment of methyl groups to other molecules in order to catalyse many important cellular functions in the body (Figure 1). Methylation is vital to life as we know it, controlling many important functions such as epigenetic control of gene transcription,[1] production of adenosine triphosphate (ATP)[2] and detoxification.[3] Vitamins B2, B6, B12 and folate, as well as the amino acid serine, are highly efficient at enhancing methylation reactions throughout the body, and can provide assistance to facilitate effective methylation in those with known polymorphisms.[4]
Figure 1: Five functional categories of methylation (energy production, phospholipid synthesis, cell signalling, cell maintenance, epigenetic modification).
Background Technical Information
The process of methylation is a simple biochemical reaction, involving the attachment of methyl[†] groups to other molecules in order to catalyse important cellular functions. Methylation is vital to life as we know it, controlling, among numerous things including:
- Epigenetic control of gene transcription[5];
- Growth and development, via the regulation of DNA and RNA replication[6];
- The production of ATP (energy production) [7];
- The function of the nervous system[8],[9];
- Detoxification[10]; and
- Immune function.[11]
5,10 methylenetetrahydrofolate reductase (MeTHFR)…is subject to SNPs, which can radically affect its activity and affects approximately 35% of the population
One of the reasons methylation may be functioning suboptimally in the presence of single nucleotide polymorphisms (SNPs or “snips”). For instance, the enzyme 5,10- methylenetetrahydrofolate reductase (MeTHFR) is responsible for conversion of folate to its active form (5-methyltetrahydrofolate, 5-MTHF). This enzyme is subject to SNPs, which can radically affect its activity and affects approximately 35% of the population.[12]
In addition, other enzymes within the folate cycle may also show genetically-mediated variation in activity, which may affect the overall competence of methylation reactions around the body. Supplementing directly with 5-MTHF may promote a more efficient folate cycle. Additionally, supplementation with the activated forms of vitamins B2 (riboflavin 5-phosphate), B6 (pyridoxal 5-phosphate) and B12 (methylcobalamin) can act as a form of ‘nutritional insurance’ against the often undetected impairments in methylation, and facilitate effective methylation in those with known polymorphisms.[13]
Serine is a non-essential, glucogenic amino acid used in the synthesis of ethanolamine and choline for phospholipids. These, in turn, are used to produce pyrimidines, purines, glycine, and the fatty myelin sheaths around nerve fibres. Serine is also needed for the removal of the pro-oxidant amino acid metabolite, homocysteine, through the transsulphation pathway (Figure 2).
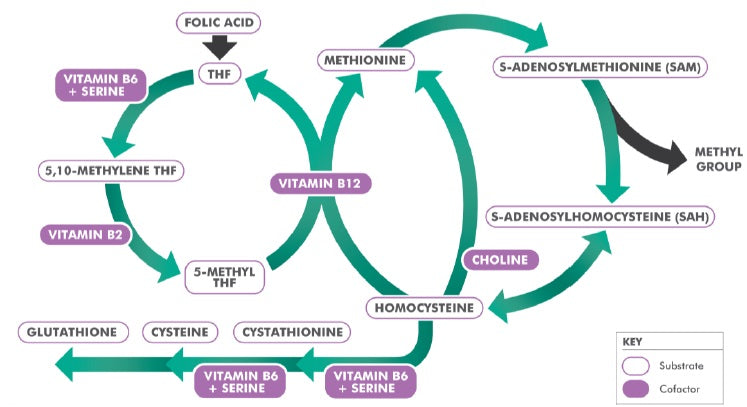
Figure 2: The methylation cycle.
Actions
Supports DNA Replication and Genomic Stability
Folate is essential for DNA synthesis, so without folate, living cells cannot divide. It plays an essential role in the production of purines and pyrimidines that make up DNA. This makes folate a critical nutrient in cell division and the repair of genetic material, and it is generally required for genomic stability. In light of folate’s fundamental role in DNA synthesis, deficiency of this nutrient impacts most significantly on those cells and tissues that exhibit a high turnover (e.g. blood cells, gastrointestinal epithelial cells), and also during those stages of development with increased growth rates, such as pregnancy and foetal tissue development.[14] Vitamin B12 is also essential for DNA synthesis and nuclear maturation, which in turn leads to normal red blood cell maturation and division.[15]
Supports Optimal Epigenetics
The genes that constitute our inherited ‘book of life’ do not have to be, and may never be, expressed. The primary influences on our genetic expression are dietary, lifestyle and other environmental factors.[16] Epigenetics is the study of how these factors influence our genetic expression, and essentially how the DNA sequence within our genome is read and expressed. Epigenetic changes can alter disease risk and ageing by influencing genetic transcription. For example, epigenetic research suggests that many cancers may not initially be caused by mutations in genes, but rather by epigenetic alterations that allow latent oncogenes to become ‘active’ and subsequently transcribed.[17] The most common mechanisms of epigenetic regulation involve:[18]
- DNA methylation;
- Histone tail modification; and
- Micro RNA (miRNA) activation.
Evidence indicates that both ageing and chronic diseases such as metabolic syndrome, neurodegeneration and cancer are associated with alterations in the epigenome. For instance, it is reported that during human ageing there is a shift towards global hypomethylation, resulting in the increased expression of ‘pro-ageing’ genes, such as oncogenes and the genes that encode for pro-inflammatory cytokines.[19] Results from several studies indicate that diet and lifestyle drive the altered methylation patterns. For example, diets low in folate and methionine, which are necessary for the methylation pathway, can lead to hypomethylation.[20]
Supports Neurotransmitter Production
There is considerable evidence for methylation cycle dysregulation in schizophrenia, bipolar disorder, depression, and autism. Low serum folate and/or cobalamin levels, as well as high plasma or serum total homocysteine is prevalent in these psychiatric conditions. In addition, epigenetic studies have demonstrated that methylation influences the expression of key genes in the brain, affecting behaviour, memory, learning and cognition. These key genes have varying degrees of reduced expression in the aforementioned patient groups.[21]
Vitamin B6 is essential for maintaining normal healthy stress levels through several mechanisms. Pyridoxal 5-phosphate (P5P) interacts with gamma-aminobutyric acid (GABA) and serotonin, and is also a cofactor in the conversion of 5-hydroxytryptophan (5HTP) to serotonin. Furthermore, P5P functions as a cofactor in the biochemical pathway whereby tyrosine is converted to epinephrine or norepinephrine, specifically in the intermediate step, where dopa is converted to dopamine. Within the brain, glutamic acid is converted to GABA via the enzyme glutamate decarboxylase and its cofactor, P5P. Studies indicate that the enhancement of GABA levels can improve relaxation and sleep.[22] Folate is implicated in cognition and neurodegeneration, due to its ability to facilitate synthesis of neurotransmitters and improve nitric oxide (NO) levels in the brain. Additionally, atrophy of the cerebral cortex results from folate deficiency.[23]
Dopamine signalling is also impacted by methylation, in particular the function of the D4 receptors. Located mainly within the prefrontal cortex (PFC), D4 receptors modulate the excitability of prefrontal neurons and are thought to be involved in working memory, impulse control and learning processes.[24],[25]
Diet and lifestyle drive altered methylation patterns.
Clinical Applications
Management of Hyperhomocysteinaemia
Homocysteine is a pro-oxidant amino acid and potentially toxic intermediate substrate in the methylation cycle. Its metabolism is catalysed by a number of enzymes that require B-vitamins as cofactors, with homocysteine levels being particularly responsive to folate status. By accepting a methyl group from the folate-cobalamin methylation pathway, homocysteine can be safely restored to methionine. By combining with serine, homocysteine is converted to cystathionine and then to further transmethylation products, namely cysteine, glutathione, taurine and finally sulfate – all valuable components of antioxidant adaptation and detoxification.
Although homocysteine is naturally occurring in the human body, in cases where methylation is impaired it accumulates and may cause disease.[26] Raised levels of homocysteine have been associated with an increased risk for many disorders, including vascular and neurodegenerative diseases, autoimmune disorders, birth defects, diabetes, renal disease, osteoporosis, neuropsychiatric disorders, chronic fatigue, and cancer.[27],[28],[29] Interestingly, a large body of data links hyperhomocysteinaemia and folate status with oxidant stress. It is suggested that homocysteine not only promotes cellular and protein injury via oxidant mechanisms, but is also a marker for the presence of pathological oxidant stress.[30]
Raised levels of homocysteine have been associated with an increased risk for many disorders
Cystathionine beta-synthase (CBS) uses P5P as a cofactor to catalyse the reaction whereby homocysteine and serine combine to form cystathionine. Deficiency of serine and/or P5P can result in a situation known as ‘the CBS block’, in which this reaction does not proceed, leading to raised homocysteine levels and increased risk of cardiovascular and neurodegenerative disorders. Additionally, impaired glutathione production impairs intra-cellular antioxidant defences and detoxification, further contributing to chronic disease risk. Providing supplemental serine and P5P can help overcome the CBS block, protecting against disease.[31]
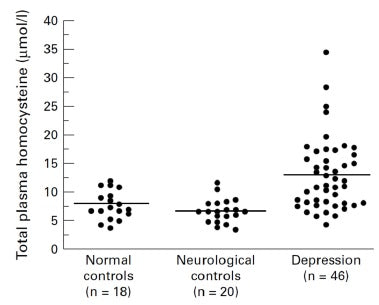
Figure 3: Total plasma homocysteine in normal controls, neurological controls and depressed subjects.[32]
Stress and Mood Support
Methylation and neurotransmitter recycling, with an emphasis on S-adenosylmethionine (SAMe) involvement, are highly dependent upon B vitamins such as folate and vitamin B12. This mechanistic understanding is reflected by clinical studies that show as many as one third of severely depressed patients manifest folate deficiency, and in these people, folate supplementation has been shown to enhance recovery of their mental state.[33]
As there are difficulties in interpreting serum and red cell folate assays in some patients, it has been suggested that total plasma homocysteine could be a more sensitive measure of functional folate and vitamin B12 deficiency.[34]A study of homocysteine, folate, and monoamine metabolism was therefore undertaken in patients with severe depression.[35] In 46 in-patients with severe depression, blood counts, serum and red cell folate, serum vitamin B12, and total plasma homocysteine were measured. Additionally, in 28 of these patients, cerebral-spinal fluid (CSF) folate, SAMe, and monoamine neurotransmitter metabolites were also examined. Twenty-four of the 46 depressed patients (52%) had raised total plasma homocysteine (Figure 3). They also displayed significantly reduced folate in their serum, red cells and CSF, and lower levels of SAMe and all three monoamine metabolites. Total plasma homocysteine was significantly negatively correlated with red cell folate in depressed patients (Figure 4), but not controls, indicating that it may be useful as a marker for a biological subgroup of depressed patients with folate deficiency, impaired methylation, and inadequate monoamine neurotransmitter metabolism.
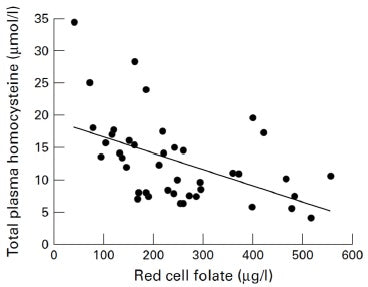
Figure 4: Relation between total plasma homocysteine and red cell folate in depression.[36]
Higher homocysteine levels have been found in people experiencing anxiety, anger, hostility, panic attacks and post-traumatic stress disorders compared to control levels.[37],[38],[39],[40] Controlled human experiments have found that plasma homocysteine levels rise during short-term stressful activities, possibly driven by the sympathetic nervous system, highlighting the link between homocysteine and stress.[41],[42] In a study of 2,682 men aged between 42 and 60 years, depressive symptoms were assessed with the 18-item Human Population Laboratory Depression Scale. Those who scored five or more at baseline were considered to have elevated depressive symptoms (n=228, 9.3% of the cohort). The participants were grouped into tertiles according to their dietary folate intake. Those in the lowest tertile of energy-adjusted folate intake had a higher risk of being depressed than those in the highest folate intake tertile. This increased risk remained significant after adjustment for smoking habits, alcohol consumption, appetite, body mass index (BMI), marital status, education, adulthood, socioeconomic status, and total fat consumption. These results indicate that nutritional folate status may play a role in the prevention of depression.[43]
One study looking at the correlation between stress and high homocysteine levels suggested a possible cause, namely that acute stress can induce a rapid decline in vitamin B6, which can in turn increases homocysteine levels.[44]Further, Vitamin B6 is directly involved in serotonin production and is particularly important in brain chemistry. As a result, a vitamin B6 deficiency may have profound effects upon mood and behaviour.[45]The results of two studies highlight the relationship between vitamin B6 and mood disorders. In one study, 140 individuals were assessed for depression and biochemical markers of B vitamin status. It was found that high depression scores were associated with low vitamin B6 status, suggesting vitamin B6 has an influence on mood.[46] In the second study, vitamin B6 levels were compared to levels of distress experienced by 135 grieving men. It was found that those with a pyridoxine deficiency scored significantly lower on the Profile of Mood States (POMS)[‡] questionnaire, compared to men with adequate pyridoxine levels (p =0.04). The authors suggest that adequate vitamin B6 levels may avert psychological distress during emotional situations, such as grieving, by regulating serotonin levels.[47]
Cardiovascular Support
Elevated levels of homocysteine have been identified as an independent risk factor for coronary artery disease.[48] Independent of its place in the methylation cycle, evidence also suggests that homocysteine is in itself a toxic compound that can directly promote atherosclerosis, neuronal damage and mood disturbances.[49] Therefore, hyperhomocysteinaemia is not only a marker of reduced methylation status, but also a direct pathogenic agent. Patients with elevated homocysteine would therefore benefit from reducing their levels.
Homocysteine is in itself a toxic compound that can directly promote atherosclerosis, neuronal damage and mood disturbances.
There has been some discussion in the literature regarding whether nutrients that support methylation, and thereby reduce serum homocysteine levels, are of any real benefit in the management of cardiovascular disease. A 2015 Cochrane Review[50] investigated this question, looking at studies where B vitamin therapy was used to lower elevated homocysteine levels, in an attempt to prevent cardiovascular events. The review found that homocysteine-lowering interventions did not significantly affect non-fatal or fatal myocardial infarction, stroke, serious adverse events (cancer) or death from any cause, compared to placebo. This result is interesting, given that elevated serum levels of homocysteine have consistently been linked to chronic disease, including cardiovascular disease, as discussed previously.[51]Such elevations in homocysteine have been linked to reduced levels of DNA methylation,[52]suggesting that while it may serve as a useful proxy for overall methylation capacity, there are additional drivers to these homocysteine-associated conditions.
This is reflected in a recent study[53] that found B vitamin therapy achieved expected reductions in homocysteine levels, yet did not reduce the levels of another important marker of methylation, S-adenosylhomocysteine (SAH).[54]Similarly, numerous studies show that folate alone or with B vitamins reduces homocysteine, yet rarely corrects DNA hypomethylation in humans.[55] In light of this, other drivers of cardiovascular disease should also be addressed in conjunction with the supplementation of folate and other methylating cofactors for the reduction of elevated homocysteine levels. These drivers include insulin resistance, inflammation, oxidative stress, endothelial damage, hormonal dysfunction and mood- and stress related issues.[56],[57],[58],[59],[60],[61],[62]
The reduction of homocysteine for cardiovascular disease management, therefore, needs to be broader than simply targeting B vitamin dependent enzymes via oral supplementation. Ultimately, what this evidence suggests, is that the whole patient and their suite of presenting signs and symptoms need to be addressed, along with elevations in homocysteine and associated methylation dysfunction.
Prescribing Notes
- Always use methylating cofactors such as B6 and B12 with 5-MTHF to support normal physiological use of folate derivatives, and to avoid potential masking of B12 deficiency.
Cautions and Contradindications
Moderate Level Cautions
- Methotrexate: Methotrexate exerts its cytotoxic effects by preventing conversion of folate to the active form needed by cells. There is some evidence that folate supplements reduce the efficacy of methotrexate in the treatment of acute lymphoblastic leukaemia, and theoretically they could reduce its efficacy in the treatment of other cancers.[63],[64] Theoretically L-5-methyl-THF may be associated with a reduced interaction with drugs that inhibit dihydrofolate reductase.[65] Seek approval from patient’s oncologist before recommending the use of folate. In patients treated with long-term, low-dose methotrexate for rheumatoid arthritis (RA) or psoriasis, folate supplements can reduce the incidence of side-effects, without reducing efficacy, but need to be dosed appropriately.[66],[67]
- Phenobarbital: Pyridoxine at doses above 200 mg daily can reduce plasma levels of the anticonvulsant phenobarbital. High doses of pyridoxine should be avoided in people on this medication.[68],[69]
- Phenytoin: Pyridoxine at doses above 200 mg daily can reduce plasma levels of the anticonvulsant phenytoin. High doses of pyridoxine should be avoided in people on this medication.[70],[71]
Low Level Cautions
- Amiodarone: Conflicting information exists regarding potential interactions between amiodarone and pyridoxine. Case reports suggest that pyridoxine could exacerbate amiodarone-induced photosensitivity. Mechanisms for this effect are unknown. Monitor patient for signs of photosensitivity.[72],[73]
Pregnancy and Breastfeeding
- Pregnancy: A review did not identify any concerns for use during pregnancy, however safety has not been conclusively established in humans.
- Lactation: Safe to use.[74],[75],[76],[77]
Footnotes
[†] A methyl group consists of a single carbon atom with three hydrogens attached (CH3).
[‡] The Profile of Mood States (POMS) is a psychological rating scale used to assess transient and distinct mood states.
MTHFR Genes
What does the MTHFR gene test result mean?
Results typically are reported as negative or positive and, if positive, the report will name the mutation(s) present.
Of the MTHFR genes, MTHFR C677T and A1298C mutations are among the most common.
If someone has two copies of MTHFR C677T, or one copy of C677T and one of A1298C this is referred to as homozygous.
If someone has one copy of MTHFR C677T or one copy of C677T this is referred to as heterozygous.
If the MTHFR mutation test is negative, the C677T and A1298C mutations were not detected.
Other, rarer MTHFR genetic mutations will not be detected with typical testing.
Can my MTHFR genes change?
No, you inherit a copy of the gene from each of your parents and they will not change over time.
What does MTHFR genetic mutations cause?
MTHFR genetic mutations can reduce the efficiency of your body to convert folic acid into the active forms of folic acid via the Methylation process.
This can lead to elevated homocysteine levels.
People who have elevated homocysteine levels may be at an increased risk of developing premature cardiovascular disease (CVD) and/or thrombosis, but many, including those with MTHFR mutations, will never develop CVD or thrombosis. The role of homocysteine in cardiac risk assessment is still in the process of being determined.
As well as MTHFR mutations there are other causes of elevated homocysteine levels including deficiency of vitamins B6, B12, and/or folate – vitamins required for homocysteine metabolism, kidney and other diseases, numerous drugs, and increasing age. Also, if a rarer mutation of MTHFR is causing elevated homocysteine levels, the C677T and A1298C tests will not detect these other mutations.
Some studies have shown links between MTHFR genetic mutations and an increased risk of neural tube defects, miscarriage and pre-eclampsia, and certain cancers.
The MTHFR enzyme is involved in folate metabolism. Because of this, those who have MTHFR mutations and take drugs that affect folate metabolism, such as methotrexate, may be more likely to experience toxicity. An MTHFR mutation test may be performed for a person who is prescribed methotrexate in order to adjust dosages and reduce risk of toxicity although this is not routinely done.
Those with MTHFR mutations and other clotting risk factors, such as Factor V mutation (Leiden) or PT 20210 mutations, may be at an increased risk of thrombosis.
If I have MTHFR genetic mutations should I take folic acid or activated folic acid (folinic acid or 5-Methylhydrafolate)?
References
[1] Nazki FH, Sameer AS, Ganaie BA. Folate: metabolism, genes, polymorphisms and the associated diseases. Gene. 2014 Jan 1;533(1):11-20.
[2] Joncquel-Chevalier Curt M, et al. Creatine biosynthesis and transport in health and disease. Biochimie. 2015 Dec;119:146-65.
[3] Hoffman M. Hypothesis: Hyperhomocysteinemia is an indicator of oxidant stress. Medical Hypotheses. 2011;77:1088–1093.
[4] Gropper S, Smith JL. Water soluble vitamins. In: Advanced nutrition and human metabolism. 6th ed. Australia: Wadsworth, Cengage Learning. 2013:307-69.
[5] Nazki FH, Sameer AS, Ganaie BA. Folate: metabolism, genes, polymorphisms and the associated diseases. Gene. 2014 Jan 1;533(1):11-20.
[6] Crider KS, et al. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr. 2012 Jan;3(1):21-38.
[7] Joncquel-Chevalier Curt M, et al. Creatine biosynthesis and transport in health and disease. Biochimie. 2015 Dec;119:146-65.
[8] Sugden C. One-carbon metabolism in psychiatric illness. Nutrition Research Reviews. 2006;19:117–136.
[9] Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern Med Rev. 2008 Sep;13(3):216-26.
[10] Hoffman M. Hypothesis: Hyperhomocysteinemia is an indicator of oxidant stress. Medical Hypotheses. 2011;77:1088–1093.
[11] Nestor CE, Barrenäs F, Wang H, et al. DNA methylation changes separate allergic patients from healthy controls and may reflect altered CD4+ T-cell population structure. PLoS Genet. 2014 Jan;10(1):e1004059.
[12] Botto LD, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol. 2000;151(9):862-77.
[13] Gropper S, Smith JL. Water-soluble vitamins. In: Advanced nutrition and human metabolism. 6th ed. Australia: Wadsworth, Cengage Learning. 2013:307-69.
[14] Braun L, Cohen M. Folate. In: Herbs and natural substances: an evidence-based guide. 4th ed. Vol 2. Sydney: Elsevier/Churchill Livingstone. 2015:364-80.
[15] Gaspard KJ, Porth CM, Matfin G, editors. Ch 14: Disorders of the Haemopoietic System. In: Pathophysiology, concepts of altered health states. 8th ed. Philadelphia PA. Lippincott, Williams & Wilkins. 2009:290-291.
[16] King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643-646.
[17] Issa JP. Epigenetic variation and human disease. J Nutr. 2002;132(8 Suppl):2388S-2392S.
[18] Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nut. 2002;132(8 Suppl) 2393S–2400S.
[19] Fenech M, El-Sohemy A, Leah C, et al. Nutrigenetics and nutrigenomics: viewpoints on the current status and applications in nutrition research and practice. J Nutrigenet Nutrigenomics. 2011;4(2):69-89.
[20] Berdasco M, Esteller M. Hot topics in epigenetic mechanisms of aging: 2011. Aging Cell. 2012 Apr;11(2):181-6.
[21] Sugden C. One-carbon metabolism in psychiatric illness. Nutrition Research Reviews. 2006;19:117–136.
[22] Head K, Kelly G. Nutrients and botanicals for treatment of stress: adrenal fatigue, neurotransmitter imbalance, anxiety and restless sleep. Alternative Medicine Review. 2009;14(2);114-140.
[23] Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008 Oct 8;(4):CD004514.
[24] Tripp G, Wickens JR. Neurobiology of ADHD. Neuropharmacology. 2009 Dec;57(7-8):579-89.
[25] Sugden C. One-carbon metabolism in psychiatric illness. Nutrition Research Reviews. 2006;19:117–136.
[26] Hoffman M. Hypothesis: Hyperhomocysteinemia is an indicator of oxidant stress. Medical Hypotheses. 2011;77:1088–1093.
[27] Splaver A, Lamas GA, Hennekens CH. Homocysteine and cardiovascular disease: biological mechanisms, observational epidemiology, and the need for randomized trials. Am Heart J. 2004 Jul;148(1):34-40.
[28] Regland B, Andersson M, Abrahamsson L. Increased concentrations of homocysteine in the cerebrospinal fluid in patients with fibromyalgia and chronic fatigue syndrome. Scand J Rheumatol. 1997;26:301-7.
[29] Brustolin S, Giugliani R, Félix TM. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010 Jan;43(1):1-7.
[30] Hoffman M. Hypothesis: Hyperhomocysteinemia is an indicator of oxidant stress. Medical Hypotheses. 2011;77:1088–1093.
[31] Gropper S, Smith JL. Advanced nutrition and human metabolism. 6th ed. Australia: Wadsworth, Cengage Learning. 2013.
[32] Bottiglieri T, Laundy M, Crellin R, et al. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000 Aug;69(2):228-32.
[33] Bottiglieri T, Laundy M, Crellin R, et al. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000 Aug;69(2):228-32.
[34] Bottiglieri T, Laundy M, Crellin R, et al. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000 Aug;69(2):228-32.
[35] Bottiglieri T, Laundy M, Crellin R, et al. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000 Aug;69(2):228-32.
[36] Bottiglieri T, Laundy M, Crellin R, et al. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000 Aug;69(2):228-32.
[37] Fraguas R Jr, Papakostas GI, Mischoulon D, et al. Anger attacks in major depressive disorder and serum levels of homocysteine. Biol Psychiatry. 2006 Aug 1;60(3):270-274.
[38] Meier C, Harbrecht U, Liedtke R, et al. Relative hyperhomocysteinemia in patients with panic disorder: a case-control study. Neuropsychobiology. 2010 Aug;62(3):164-170.
[39] Levine J, Timinsky I, Vishne T, et al. Elevated serum homocysteine levels in male patients with PTSD. Depress Anxiety. 2008;25(11):E154-7.
[40] Stoney CM, Engebretson TO. Plasma homocysteine concentrations are positively associated with hostility and anger. Life Sci. 2000;66:2267–2275.
[41] Stoney CM, Engebretson TO. Plasma homocysteine concentrations are positively associated with hostility and anger. Life Sci. 2000;66:2267–2275.
[42] Sawai A, Ohshige K, Kura N, et al. Influence of mental stress on the plasma homocysteine level and blood pressure change in young men. Clin Exp Hypertens.2008 Apr;30(3):233-41.
[43] Tolmunen T, Voutilainen S, Hintikka J, et al. Dietary folate and depressive symptoms are associated in middle-aged Finnish men. J Nutr. 2003;133(10):3233-6.
[44] Stoney CM. Plasma homocysteine levels increase in women during psychological stress. Life Sci. 1999;64(25):2359-2365.
[45] Braun L, Cohen M. Vitamin B6. In: Herbs and natural substances: an evidence-based guide. 4th ed. Vol 2. Sydney: Elsevier/Churchill Livingstone. 2015:1079-91.
[46] Hvas AM, Juul S, Bech P, et al. Vitamin B6 level is associated with symptoms of depression. Psychotherapy and Psychosomatics. 2004;73(6):340.
[47] Baldewicz T, Goodkin K, Feaster DJ, et al. Plasma pyridoxine deficiency is related to increased psychological distress in recently bereaved homosexual men.Psychosom Med. 1998 May-Jun;60(3):297-308.
[48] Brustolin S, Giugliani R, Félix TM. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010 Jan;43(1):1-7.
[49] Ordovás JM, Smith CE. Epigenetics and cardiovascular disease. Nat Rev Cardiol. 2010 Sep;7(9):510-9.
[50] Martí-Carvajal AJ, Solà I, Lathyris D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2015 Jan15;1:CD006612.
[51] Mandaviya PR, Stolk L, Heil SG. Homocysteine and DNA methylation: a review of animal and human literature. Mol Genet Metab. 2014 Dec;113(4):243-52.
[52] James SJ, Melnyk S, Pogribna M, et al. Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteinerelated pathology. J Nutr. 2002 Aug;132(8 Suppl):2361S-2366S.
[53] Mandaviya PR, Stolk L, Heil SG. Homocysteine and DNA methylation: a review of animal and human literature. Mol Genet Metab. 2014 Dec;113(4):243-52.
[54] Green TJ, Skeaff CM, McMahon JA, et al. Homocysteine-lowering vitamins do not lower plasma S-adenosylhomocysteine in older people with elevated homocysteine concentrations. Br J Nutr. 2010 Jun;103(11):1629-34.
[55] Mandaviya PR, Stolk L, Heil SG. Homocysteine and DNA methylation: a review of animal and human literature. Mol Genet Metab. 2014 Dec;113(4):243-52.
[56] Younis N, Sharma R, Soran H, et al. Glycation as an atherogenic modification of LDL. Curr Opin Lipidol. 2008;19(4):378-84.
[57] Houston M. Release the Pressure: Effective interventions for the treatment of hypertension. IFM Annual International Conference. Scottsdale, AZ:2012.
[58] Williams KT, Schalinske KL. New insights into the regulation of methyl group and homocysteine metabolism. J Nutr. 2007 Feb;137(2):311-4.
[59] Stokstad EL, Chan MM, Watson JE, et al. Nutritional interactions of vitamin B12, folic acid, and thyroxine. Ann N Y Acad Sci. 1980;355:119–129.
[60] Hou Y, Hong Y, Chen W-Q, et al. Serum homocysteine concentrations of Chinese intellectuals and the influential factors concerned. Health. 2009:1:83-87.
[61] Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011 May;473:317-325.
[62] Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease. Circulation. 2006;113:1708-1714.
[63] Natural Medicines. Folic acid, folinic acid and 5-MTHF. [Online]. 2017. Available from: https://naturalmedicines.therapeuticresearch.com/databases/food,-herbs-supplements/professional.aspx?productid=1017. [Cited 07/08/17].
[64] Braun L, Cohen M. Folate. In: Herbs and natural supplements: an evidence-based guide. 4th ed. Vol 2. Sydney: Elsevier/Churchill Livingstone. 2015:364-380.
[65] Pietrzik K, Bailey L, Shane B. Folic acid and L-5-methyltetrahydrofolate: comparison of clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinet.2010 Aug;49(8):535-48. PubMed PMID: 20608755.
[66] Natural Medicines. Folic acid, Folinic acid and 5-MTHF. [Online]. 2017. Available from: https://naturalmedicines.therapeuticresearch.com/databases/food,-herbs-supplements/professional.aspx?productid=1017. [Cited 07/08/17].
[67] Braun L, Cohen M. Folate. In: Herbs and natural substances: an evidence-based guide. 4th ed. Vol 2. Sydney: Elsevier/Churchill Livingstone. 2015:364-380.
[68] Natural Medicines. Vitamin B6. [Online]. 2017. Available from: https://naturalmedicines.therapeuticresearch.com/databases/food,-herbssupplements/professional.aspx?productid=934. [Cited 07/08/17].
[69] Braun L, Cohen M. Vitamin B6. In: Herbs and natural substances: an evidence-based guide. 4th ed. Vol 2. Sydney: Elsevier/Churchill Livingstone. 2015:1078-1091.
[70] Natural Medicines. Vitamin B6. [Online]. 2017. Available from: https://naturalmedicines.therapeuticresearch.com/databases/food,-herbssupplements/professional.aspx?productid=934. [Cited 07/08/17].
[71] Braun L, Cohen M. Vitamin B6. In: Herbs and natural substances: an evidence-based guide. 4th ed. Vol 2. Sydney: Elsevier/Churchill Livingstone. 2015:1078-1091.
[72] Natural Medicines. Vitamin B6. [Online]. 2017. Available from: https://naturalmedicines.therapeuticresearch.com/databases/food,-herbssupplements/professional.aspx?productid=934. [Cited 07/08/17].
[73] Braun L, Cohen M. Vitamin B6. In: Herbs and natural substances: an evidence-based guide. 4th ed. Vol 2. Sydney: Elsevier/Churchill Livingstone. 2015:1078-1091.
[74] Braun L, Cohen M. Folate. In: Herbs and natural supplements: an evidence-based guide. 4th ed. Vol 2. Sydney: Elsevier/Churchill Livingstone. 2015:364-380.
[75] Natural Medicines. Vitamin B6. [Online]. 2017. Available from: https://naturalmedicines.therapeuticresearch.com/databases/food,-herbssupplements/professional.aspx?productid=934. [Cited 07/08/17].
[76] Natural Medicines. Vitamin B2. [Online]. 2015. Available from: https://naturalmedicines.therapeuticresearch.com/databases/food,-herbssupplements/professional.aspx?productid=957. [Cited 07/08/17].
[77] Natural Medicines. Vitamin B12. [Online]. 2017. Available from: https://naturalmedicines.therapeuticresearch.com/databases/food,-herbssupplements/professional.aspx?productid=926. [Cited 07/08/17].
