Metagenics Indole-3-Carbinol (I3C) 126g
Directions: Metagenics Indole-3-Carbinol (I3C)
1 metric teaspoon (2.1 g) is equal to 300 mg of Indole-3-Carbinol
- For oestrogen dominant conditions/to increase 2:16 hydroxyoestrone ratios – 300 mg/day
- For oestrogen-dependent cancer prevention – 300 mg/day
- For cervical dysplasia – 300 mg/day
- For support with anti-oestrogen therapy - 300 mg/day
Store below 2° C to 8° C (Refrigerate. Do not freeze.)
Clinical Benefits: Metagenics Indole-3-Carbinol (I3C)
Key Actions:
- Oestrogen detoxification – reduces the 2:16 hydroxyoestrone ratio
- Beneficial activation of the aryl hydrocarbon receptor
- Decreases oestrogen receptor alpha signalling
- Cell cycle regulation
- Antioxidant and cytoprotective – activates the NRF2 receptor
Key Applications
- Oestrogen detoxification – oestrogen dominant conditions
- Premenstrual syndrome
- Fibrocystic breast disease
- Fibroids
- Endometriosis
- Prevention of oestrogen dominant cancers
- Cervical dysplasia and cervical cancer prevention
- Support of patients using anti-oestrogen therapy
Ingredients: Metagenics Indole-3-Carbinol (I3C)
Indole-3-carbinol (I3C)
Storage: Metagenics Indole-3-Carbinol (I3C)
Store below 2° C to 8° C (Refrigerate. Do not freeze.)
Technical Information: Indole-3-carbinol (I3C)
Background Technical Information
Oestrogen Metabolism and Detoxification
The detoxification pathway through which oestrogen is metabolised will influence the overall oestrogenic load in the body. Oestrogen and its metabolites, which also have oestrogenic activity, are a beneficial and necessary part of numerous physiological functions in men and women. However, an imbalance of oestrogen metabolites, such as increased levels of more potent forms, may compromise health by increasing the risk of cancer and other hormone-dependent conditions such as endometriosis and fibrocystic breast disease.
Phase I and II Detoxification of Oestrogen
Oestrogen detoxification and elimination requires optimal function of the cytochrome P450 (CYP450) family of enzymes in phase I detoxification, where hydroxylation takes place; and also requires optimal functioning of phase II detoxification processes such as sulphation, methylation, glutathionation and glucuronidation.
Oestrogen can be metabolised via one of three hydroxylation pathways in phase I detoxification: CYP1A1/1A2, CYP1B1, and CYP3A4 (Figure 1).

Figure 1. Oestrogen metabolism and detoxification.
- CYP1A1/1A2 converts oestrogens to 2-hydroxyoestrogens (2-OHO), which are antiproliferative. These can undergo methylation to produce the protective 2-methoxyoestrogens (2-MeO). 2- methoxyoestrone is a powerful protective oestrogen; it has been found to have anti-proliferative actions, to inhibit aromatase, and has been considered for treatment of refractory breast cancer.1
- CYP1B1 is active within target tissues and converts local oestrogen to 4-hydroxyoestrogens (4- OHO), whose proliferative actions are thought to be restricted to nearby cells. For instance, 4-OHO has been found to be involved in the initiation of breast cancer within the breast tissue, however does not appear to reach the blood and exert systemic effects.2,3
- CYP3A4 enzymes form the 16-hydroxyoestrogens (16-OHO); which are highly metabolically active systemically and potently proliferative oestrogens.4
Quinone Formation
2-OHO and 4-OHO can create DNA-damaging quinones if they are not successfully methylated to 2-MeO and 4-MeO oestrogens, respectively. Quinones are organic compounds that can form superoxide and hydroxyl radicals and cause single strand DNA breaks; these DNA breakages are thought to be one of the first stages of cancer initiation.5,6
The 2:16 Hydroxyoestrone Ratio
In vitro and animal studies have indicated that 16-hydroxyoestrone (16-OHE1) has proliferative effects in breast cancer, while the 2-hydroxyoestrone metabolites have protective effects. Therefore, a low ratio of 2- hydroxyoestrone to 16-hydroxyoestrone is thought to indicate an increased risk of breast cancer*.7 Recent studies have found that women with breast cancer often have a 2:16 ratio below 2.0, which further supports this hypothesis.8Hormone replacement therapy can increase the amount of 4-OH oestrogens, and certain drugs, lifestyle factors such as physical activity and cigarette smoking, as well as genetic polymorphisms affecting key enzymes, may also have an impact on oestrogen metabolism.9
Actions
Oestrogen Detoxification – Reducing the 2:16 Ratio
Both human clinical trial and in vitro research supports the use of I3C in promoting healthy oestrogen detoxification. I3C administration in breast cancer cell lines induced a four- to ten-fold increase in 2- hydroxylation in oestrogen responsive cells, but not in the non-oestrogen responsive cell lines tested. This increase in 2-hydroxylation in oestrogen responsive cells was via the induction of CYP1A1 enzymes.10 In human clinical trials, I3C has been shown to provide beneficial effects on the 2:16 hydroxyoestrone ratio in as little as four weeks (see Applications section).
I3C More Effective than DIM
Upon contact with gastric acid, oral I3C undergoes rapid conversion to a number of condensation products, the major one being 3,3’diindolylmethane (DIM). Other condensation products include ascorbigen, cyclic trimer (CTR), linear trimer (LTR), and indolo[3,2-b]carbazole (ICZ), all of which are produced in varying amounts and may contribute to the biological activity of I3C.11 These compounds, particularly DIM and ICZ, are thought to be responsible for the long-term anti-oestrogenic biological activities of supplemental I3C, which may contribute to protection against mammary tumour formation.12
When comparing the efficacy and safety of oral I3C and oral DIM supplementation, there are a larger number of clinical trials for I3C demonstrating beneficial effects on hormone markers, with limited evidence suggesting that DIM has a similar effect. In a number of clinical studies, supplementation with I3C has been shown to increase the 2:16 hydroxyoestrone ratio.. In the only trial available to date for DIM, supplementation with DIM led to a favourable, although not statistically significant, shift in the 2:16 ratio.13,14
Oestrogen Detoxification – Activation of Aryl Hydrocarbon Receptor
A large body of evidence supports the use of I3C as an anticarcinogenic compound both in vitro and in vivo. 15,16, 17,18 I3C has been shown to exert its anti-cancer effects primarily by influencing a number of transcription factors which are involved in the regulation of cellular apoptosis and proliferation, including aryl hydrocarbon receptors (AhR) and oestrogen receptors (ER) (Figure 2).19
* Urinary 2:16 hydroxy-oestrogen ratios below 2.0 are considered to be a risk factor for oestrogen-dependent cancers, such as breast and uterine cancer. A ratio below 1.6 is associated with an even greater increase in oestrogen-dependent conditions. Ideally, levels should be above 2.0 or preferably above 2.4, if there is a history of oestrogen-dependent cancers. However a high 2:16 ratio, above 3.0, may be considered a risk of developing oestrogen deficient conditions such as menopausal symptoms and osteoporosis.

Figure 2. Biological effects of I3C.20
AhR is a ligand-activated transcription factor which binds to response elements inside the promoter regions of target genes.21 Activation of AhR-mediated pathways increases the expression of genes involved in metabolising xenobiotic compounds and oestrogen. I3C is a ligand for AhR, which upregulates the transcription of Ah-responsive genes such as CYP1A1.22 Increased CYP1A1 activity is associated with positive improvements in oestrogen metabolism due to increased hydroxylation of E1 and E2 to form less proliferative 2-OH oestrogens (refer to Figure 1). More specifically, administration of I3C resulted in increased mRNA for CYP1A1 and CYP1A2 by 3.8 and 2.2 times, respectively.23
Activation of the aryl hydrocarbon receptors not only upregulates CYP1A1/CYP1A2 enzymes (which is beneficial in promoting the metabolism of E1 and E2 into 2-OH oestrogen), but also CYP1B1.24,25 While unregulated increase in CYP1B1 activity may raise concerns due to the potential formation of 4-OH oestrogen,26,27 I3C has been shown to improve the CYP1A1/1B1 expression ratio and provide anti- proliferative effects in breast cancer cells.
Combine I3C with Phase II Detoxification Support
To ensure the complete transformation of phase I oestrogens into inactive metabolites, it is recommended to prescribe I3C in conjunction with adequate levels of the methylating nutrients such as vitamins B6, B12 and folinic acid. Healthy Hormone Balance is an ideal companion formula to I3C as it contains turmeric, which induces glutathione-S-transferase (GST), an enzyme responsible for inhibiting quinone production,28,29 as well as activated folate (folinic acid), vitamin B6 and vitamin B12, which are cofactors for catechol-O- methyltransferase (COMT) – the enzyme responsible for converting 2- and 4-OHE1 oestrogens to their inactive metabolites 2- and 4-methoxyoestrone.
Reducing Oestrogen Signalling – Modulation of Oestrogen Receptor Alpha
Oestrogen receptor alpha (ERα), another target of I3C, is an important target for strategies to control oestrogen-dependent cancer proliferation, as the expression of ERα has been associated with increased tumour progression.30 I3C may influence oestrogen signalling via a number of mechanisms, including suppressing ERα induced gene expression,31 reducing the expression of ERα proteins (possibly via an AhR- mediated mechanism)32,33 and by altering the extent of oestrogen binding to ERα.
Cell Cycle Regulation
As seen in Figure 2, I3C has effects beyond hormone metabolism on cell cycle regulation which may play a role in cancer prevention. Studies have found that I3C may induce apoptosis and cell cycle arrest in breast and prostate cancer cells via the alteration of intracellular kinase signalling.35,36
In the cell cycle, the Gap 1 (G1) phase is the first of four phases of the cell cycle that takes place in eukaryotic cell division. In G1, the cell grows in size and synthesises mRNA and proteins in preparation for subsequent steps leading to mitosis. G1 is a key target to alter the proliferation rate of normal and transformed mammary epithelial cells. I3C has been shown to induce G1 cell cycle arrest in cultured human MCF7 breast cancer cells, an effect which may be due to reduced transcription and activity of cyclindependent kinase-6 (CDK6) cell cycle components.37
In vitro evidence showed that the administration of I3C to invasive human breast cancer cells significantly suppressed cell attachment, spreading, and invasion. The extent of breast cancer metastasis depends on the ability of the cell to adhere to the basement membrane, and to invade through the extracellular matrix and migrate away from the primary tumour environment.38
Antioxidant and Cytoprotection – Activation of Nrf2 Receptor
Excessive oxidative stress can cause DNA damage, which drives genomic instability and ultimately tumourogenesis. Additionally, xenobiotic compounds, many with oestrogenic activity, can cause oxidative stress and are linked to the development of cancers. As a cytoprotective defensive mechanism to combat xenobiotic and subsequent oxidative damage, cells have various protective enzymes to counteract oxidative stress and detoxify carcinogenic compounds.39
Animal studies have investigated the effect of I3C on xenobiotic metabolism and induction of endogenous antioxidant enzymes as a mechanism for prevention of carcinogenesis. I3C administration resulted in a significant increase in quinone reductase, GST, and UDP-GT, important phase II detoxification enzymes. The authors suggested that this may be in part due to I3C’s ability to activate nuclear factor (erythroid- derived 2)-like 2 (Nrf2), but also due to its action as a ligand for AhR. Nrf2 is the intracellular defence mechanism against oxidative stress and xenobiotic exposure, as it regulates the expression of genes for phase II xenobiotic metabolic enzymes and engoenous antioxidant enzymes. The Nrf2-antioxidant response element (ARE) signalling pathway has been shown to play an important role in cancer chemoprevention.40
In the cytoplasm of the cell, Nrf2 is bound to inhibitory protein Kelch-like ECH-associated protein-1 (Keap 1). Exposure to oxidative stress or chemical inducers (i.e. phytochemicals) leads to the release of Nrf2 from its anchor protein and subsequent translocation into the nucleus where it binds to ARE in the promoter region of target genes (Figure 3). This, in turn, upregulates the expression of phase II detoxifying genes and protective antioxidant genes.41
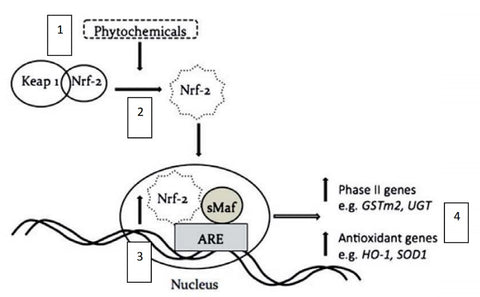
Figure 3. Schematic diagram of the proposed simplified pathway shows indole phytochemicals inducing Nrf2‐ARE signalling through activation of the ARE and producing antioxidant and phase II detoxifying genes.42
- Nrf2 is induced by exposure to oxidative stress or chemical inducers (phytochemicals).
- This leads to the release of Nrf2 from its anchor protein, Keap 1, and subsequent translocation into the nucleus.
- Here it binds to ARE in the promoter region of target genes.
- This, in turn, upregulates the expression of phase II detoxifying genes and protective antioxidant genes.
Applications
Oestrogen Detoxification
A trial was conducted to assess the efficacy of I3C in 17 women (1 postmenopausal and 16 premenopausal) from a high risk breast cancer cohort. After a four week placebo run- in period, subjects were administered 400 mg I3C daily for four weeks followed by a four week period of 800 mg I3C daily. Urinary 2:16-hydroxyoestrone ratios were measured near the end of the placebo and dosing periods. There was a 66% increase in the urinary 2:16 ratio in response to I3C, with the maximal increase observed with the 400 mg daily dose and no further increase found at 800 mg daily (figure 4). The authors concluded that 400 mg I3C daily would therefore elicit a maximal protective effect.43

In another study, a double-blind, placebo-controlled trial investigated the effects of a herbal formula including 200 mg of I3C for a period of 28 days in premenopausal women not using hormonal contraception. The treatment resulted in a significant increase in both 2-OH oestrogen concentration and 2:16-OHO ratio (p=0.003 and 0.016 respectively).45
Cervical Dysplasia and Cervical Cancer Prevention
The majority of clinical studies on I3C have been in the area of human papillomavirus (including cervical cancer and respiratory papillomatosis) with four published studies consisting of a total of 79 subjects. In these studies, 200 to 400 mg of oral I3C was given daily for 12 weeks (shortest) to 82 months (6.8 years) (longest). In these studies, 30% to 100% of subjects experienced remission or regression of their symptoms.46
In one study, thirty patients with biopsy-proven cervical intraepithelial neoplasia (CIN) II-III were randomised to receive placebo or 200, or 400 mg/day I3C for 12 weeks. There was a statistically significant regression of CIN in patients treated with I3C compared with placebo. None of the patients in the placebo group had complete regression of CIN. The main outcome measured, the 2/16-hydroxyoestrone ratio changed in a dose dependent manner (Figure 5).47
Support of Patients Undergoing Anti-Oestrogen Therapy
Tamoxifen is used clinically as an anti-oestrogen for breast cancer patients, where it acts as a competitive inhibitor of oestradiol binding to the oestrogen receptor. While tamoxifen has been shown to decrease the activity of the oestrogen receptor, it does not however have an antiproliferative effect on oestrogen receptor- negative lines. In contrast, studies show that I3C can suppress the growth of cells regardless of oestrogen receptor status, which may be due to its cell-regulating and protective actions described earlier.49
Additionally, I3C has been shown to act synergistically with tamoxifen. The combination of tamoxifen and I3C caused a more potent growth arrest in the oestrogen-dependent human MCF-7 breast cancer cell lines than either compound alone. I3C works through a mechanism distinct from tamoxifen. I3C does not compete with oestrogen for oestrogen receptor binding and instead specifically down-regulated the expression of CDK6; a protein important for regulating cell cycles. These findings demonstrate that I3C and tamoxifen work through different signal transduction pathways to suppress the growth of human breast cancer cells (Figure 6). I3C can therefore be used as a potential adjuvant therapeutic for oestrogen-responsive breast cancer.50

Figure 5. Left - regression of CIN compared to dose of indole-3-carbinol; right - change in oestradiol metabolism in placebo versus control.48

Figure 6. Model for the comparison of the antiproliferative effects of I3C and tamoxifen in breast cancer cells.51
I3C potentially mediates its effects through a putative cellular target (“target”) which is suggested to inhibit CDK6 expression and activity, and decrease the activity of CDK2. The inhibition of these two cell cycle promoting proteins is thought to decrease retinoblastoma protein (Rb) phosphorylation, which is a key promoter of cell growth. In contrast, tamoxifen mediates its effects by inhibiting the activity of the oestrogen receptor, which prevents the oestrogen-stimulated growth of breast cancer cells through a pathway that eventually converges on the activity of CDK2.
Cautions and Contraindication: Metagenics Indole-3-Carbinol (I3C)
Contraindications
- Nil
Cautions
- Oral contraceptive pill: Indole-3-carbinol may reduce the effects of the OCP due to its anti- oestrogenic action. Use with caution.
- Drugs metabolised by CYP1A1/1A2: Theoretically I3C may increase the metabolism of CYP1A1/1A2 substrates and lower or raise serum concentrations of active drug metabolites. Some substrates of CYP1A1/1A2 include clozapine, cyclobenzaprine, fluvoxamine, haloperidol, imipramine, mexiletine, olanzapine, propranolol, tacrine, theophylline, zileuton and zolmitriptan.52 Use with caution and monitor drug effects.
-
Methylation defects: When prescribing I3C, it is important to be aware that I3C works on certain metabolic pathways which form intermediate oestrogen by-products. It is recommended that I3C be used in conjunction with sufficient levels of methylating nutrients such as folinic acid, vitamin B6 and B12 to ensure that 2-OH and 4-OH are adequately methylated to form inactive metabolites. This may be particularly relevant to patients with methylation defects, such as MTHFR polymorphisms.
Pregnancy and Lactation
- Not applicable for use during pregnancy and lactation. There is insufficient data to recommend indole-3-carbinol in greater amounts than consumed in the diet. Avoid using.53
References: Metagenics Indole-3-Carbinol (I3C)
1 Mueck AO, Seeger H. Breast cancer: are oestrogen metabolites carcinogenic? Maturitas 2007;57(1):42-6.
2 Mueck AO, Seeger H. Breast cancer: are oestrogen metabolites carcinogenic? Maturitas 2007;57(1):42-6.
3 Cavalieri E, Chakravarti D, Guttenplan J, et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta. 2006 Aug;1766(1):63-78.
4 Jones MD, Ed. Textbook of Functinal Medicine. Gig Harbour WA; Institute of Functional Medicine, 2005:218-219.
5 Cavalieri E, Chakravarti D, Guttenplan J, et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta. 2006 Aug;1766(1):63-78.
6 Bland JS. Improving health outcomes through nutritional support for metabolic biotransformation. Metagenics educational programs. 2003:111.
7 Brooks JD, Ward WE, Lewis JE, et al. Supplementation with flaxseed alters estrogen metabolism in postmenopausal women to a greater extent than does supplementation with an equal amount of soy. Am J Clin Nutr 2004;79(2):318-325.
8 Haggans CJ, Travelli EJ, Thomas W, et al. The effect of flaxseed and wheat bran consumption on urinary estrogen metabolites in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2000;9(7):719-725.
9 Mueck AO, Seeger H. Breast cancer: are oestrogen metabolites carcinogenic? Maturitas 2007;57(1):42-46.
10 Ashok BT, Chen Y, Liu X, Bradow HL, et al. Abrogation of estrogen-mediated cellular and biochemical effects by indolee-3-carbinol. Nutrition and Cancer. 2001;41(1&2):180-187.
11 Bradlow HL. Indole-3-carbinole as a chemoprotective agent in breast & prostate cancer. In Vivo. 2008;22:441-6.
12 Cover CM, et al. Indole-3-carbinol and tamoxifen cooperate to arrest the cell cycle of MCF-7 human breast cancer cells. Cancer Research 1999;59:1244-1251
13 Rogan E. The natural chemopreventive compound Indole-3-carbinol: state of the science. In Vivo 2006;20:221-228
14 Minich DM, Bland JS. A review of the clinical safety and efficacy of cruciferous vegetable phytochemicals. Nutrition Reviews 2007;65(6):259-267
15 Sundar SN, et al. Indole-3-carbinol selectively uncouples expression and activity of oestrogen receptor subtypes in human breast cancer cells. Molecular Endocrinology. 2006;20:3070-82.
16 Covert CM, Hsieh SJ, Tran SH, Hallden G, Kim GS, Bjeldanes LF, et al. Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a GF1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling. J Biol Chem. 1998;273(7):3838-47.
17 Rahman KM, et al. Therapeutic intervention of experimental breast cancer bone metastasis by indole-3-carbinol in SCID-human mouse model. Mol Cancer Ther. 2006;5(11):2747-56.
18 Kim YS, Milner JA. Targets for indole-3-carbinol in cancer prevention. J Nutr Biochemistry. 2005;16:65-73.
19 Kim YS, Milner JA. Targets for indole-3-carbinol in cancer prevention. J Nutr Biochemistry. 2005;16:65-73.
20 Rogan EG. The natural chemopreventive compound Indole-3-carbinol: state of the science. In vivo 2006;20:221-228
21 Swedenborg E, Pongratz I. Ahr and ARNT modulate ER signaling. Toxicology. 2010;268:132-8.
22 Kim YS, Milner JA. Targets for indole-3-carbinol in cancer prevention. J Nutr Biochemistry. 2005;16:65-73.
23 Trusov NV, Guseva GV, Aksenov IV, et al. Effects of combined treatment with resveratrol and indole-3-carbinol. Bulletin of Experimental Biology and Medicine. 2010;149(2):174-179.
24 Gao X et al. Endocrine disruption by indole-3-carbinol and Tamoxifen: blockage of ovulation. Toxicol Appl Pharmacol 2002;183:179-188
25 Hermann S et al. Indolo{3.2-b}carbazole inhibits gap junctional intercellular communication in rat primary hepatocytes and acts as a potential tumour promoter. Carcinognesis 2002;;23(11):1861-1868
26 Cribb AE et al. Role of polymorphic human cytochrome P450 enzymes in estrone oxidation. Cancer Epidemiol Biomarkers Prev 2006;15:551-558
27 Ociepa-Zawal M et al. The effect of indole-3-carbinol on the expression of CYP1A1, CYP1B1 and AhR genes and proliferation of MCF-7 cells. Acta Biochimica Polonica 2007;54(1):113-117
28 Montano MM, Katzenellenbogen BS. The quinine reductase gene: a unique oestrogen receptor regulated that is activated by antioestrogens, Proc Natl Acad Sci USA 1997;94:2581-2586
29 Sharma RA, et al. Effects of dietary curcumin on glutathione-S-transferase and malondialdehyde-DNA adducts in rat liver and colon mucosa: relationship with drug levels. Clinical Cancer Research 2001;7:1452-1458
30 Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116(3):561-570.
31 Suborn KJ, Fan S, Rosen EM, Goodwin L, Chandraskaren A, Williams DE, et al. Indole-3-carbinol is a negative regulator of estrogen. J Nutr. 2003;133:2470S5S.
32 Sundar SN, et al. Indole-3-carbinol selectively uncouples expression and activity of oestrogen receptor subtypes in human breast cancer cells. Molecular Endocrinology. 2006;20:3070-82.
33 Wang TTY, Milner MF, Milner JA, Kim YS. Estrogen receptor alpha as a target for indole-3-carbinol. J Nutr Biochem. 2006;17(10):659-64.
34 Kim YS, Milner JA. Targets for indole-3-carbinol in cancer prevention. J Nutr Biochemistry. 2005;16:65-73.
35 Sarkar FH, Li Y. Indole-3-carbinol and prostate cancer. J Nutr. 2004;134:3493S-8S.
36 Weng J, et al. A potent indole-3-carbinol-derived antitumour agent with pleiotropic effects on multiple signalling pathways in prostate cancer cells. Cancer Res. 2007;67(16):7815-24.
37 Sundar SN, et al. Indole-3-carbinol selectively uncouples expression and activity of oestrogen receptor subtypes in human breast cancer cells. Molecular Endocrinology. 2006;20:3070-82.
38 Meng Q, Goldberg ID, Rosen EM, et al. Inhibitory effects of indole-3-carbinol on invasion and migration in human breast cancer cells. Breast Cancer Research and Treatment. 2000;63:147-152.
39 Saw C, Cintron M, Wu T, et al. Pharmacodynamics of dietary phytochemical indoles I3C and DIM: Induction of NrF2-mediated phase II drug metabolising and antioxidant genes and synergism with isothiocyanates. Biopharm. Drug Dispos. 2011; 32: 289-300.
40 Trusov NV, Guseva GV, Aksenov IV, et al. Effects of combined treatment with resveratrol and indole-3-carbinol. Bulletin of Experimental Biology and Medicine. 2010;149(2):174-179.
41 Saw C, Cintron M, Wu T, et al. Pharmacodynamics of dietary phytochemical indoles I3C and DIM: Induction of NrF2-mediated phase II drug metabolising and antioxidant genes and synergism with isothiocyanates. Biopharm. Drug Dispos. 2011; 32: 289-300.
42 Saw C, Cintron M, Wu T, et al. Pharmacodynamics of dietary phytochemical indoles I3C and DIM: Induction of NrF2-mediated phase II drug metabolising and antioxidant genes and synergism with isothiocyanates. Biopharm. Drug Dispos. 2011; 32: 289-300.
43 Reed G, Peterson K, Smith H, et al. A phase I study of indole-3-carbinol in women: tolerability and effects. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1953-60
44 Reed G, Peterson K, Smith H, et al. A phase I study of indole-3-carbinol in women: tolerability and effects. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1953-60
45 Laidlaw M, Cockerline CA, Sepkovic DW. Effects of a breast-health herbal formula supplement on estrogen metabolism in pre-and post-menopausal women not taking hormonal contraceptives or supplements: A randomized controlled trial. Breast Cancer: Basic and Clinical Research. 2010;4:85-95.
46 Minich DM & Bland JS. A review of the clinical safety and efficacy of cruciferous vegetable phytochemicals. Nutrition Reviews 2007;65(6):259-267
47 Bell MC. Placebo-controlled trial of Indole-3-carbinol in the treatment of CIN. Gynecologic Oncology 2000;78(2):123-129
48 Bell MC. Placebo-controlled trial of Indole-3-carbinol in the treatment of CIN. Gynecologic Oncology 2000;78(2):123-129
49 Cover CM, et al. Indole-3-carbinol and tamoxifen cooperate to arrest the cell cycle of MCF-7 human breast cancer cells. Cancer Research 199;59:1244-1251
50 Cover CM, et al. Indole-3-carbinol and tamoxifen cooperate to arrest the cell cycle of MCF-7 human breast cancer cells. Cancer Research 199;59:1244-1251
51 Cover CM, et al. Indole-3-carbinol and tamoxifen cooperate to arrest the cell cycle of MCF-7 human breast cancer cells. Cancer Research 199;59:1244-1251
52 http://naturaldatabase.therapeuticresearch.com/nd/Search.aspx?cs=&s=ND&pt=100&id=1027&ds=&name=indole+3+Carbinol+(INDOLE-3-CARBINOL)&searchid=40654628 date accessed 11/04/13
53 http://naturaldatabase.therapeuticresearch.com/nd/Search.aspx?cs=&s=ND&pt=100&id=1027&ds=&name=indole+3+Carbinol+(INDOLE-3-CARBINOL)&searchid=40654628 date accessed 11/04/13



